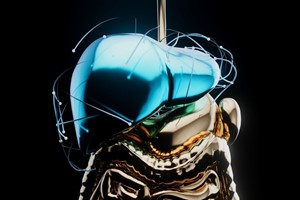
Hepatitis B virus (HBV) and hepatitis D virus (HDV) infections pose significant threats to global health, contributing to liver-related ailments such as cirrhosis and hepatocellular carcinoma. Despite strides in treatment, these viruses continue to challenge medical researchers. However, a recent breakthrough published in Nature Communications sheds light on a promising avenue for therapy: the structural basis of the interaction between a viral peptide drug and its cellular receptor.
The study, led by Professor Kaspar Locher of IMBB, ETH Zurich, in collaboration with esteemed research groups worldwide, including the Kossiakoff group (The University of Chicago), the Urban group (Heidelberg University), the Glebe group (Justus Liebig University Giessen), and the Geyer group (Justus Liebig University Giessen), elucidates the intricate mechanism by which the viral peptide drug, bulevirtide (BLV), binds to the sodium taurocholate co-transporting polypeptide (NTCP), a receptor crucial for HBV/HDV infection.
NTCP, primarily involved in the enterohepatic circulation of bile salts, was identified as the receptor for HBV/HDV. Previous research established that the N-terminal myristoylated preS1 domain of the large surface protein of HBV/HDV mediates the viral interaction with NTCP. However, the precise mechanism remained elusive until now.
Through a combination of cryo-electron microscopy (cryo-EM) and functional analysis, the research team captured the interaction between NTCP and BLV with unprecedented detail. The 3.4 Å resolution EM density map revealed the structural arrangement of NTCP, BLV, and a specific antigen-binding antibody fragment (Fab) designated Fab3.
The study uncovered that BLV inhibits HBV/HDV infection by interacting with NTCP at multiple levels. The myristoyl group of BLV engages with specific regions of NTCP, while the peptide itself adopts a globular shape, forming a plug within the translocation tunnel and extending across the membrane bilayer. This unique binding configuration disrupts the viral entry process, providing a molecular basis for BLV's potent antiviral activity.
Furthermore, the research provides insights into the functional consequences of NTCP mutations, such as the S267F mutation, which compromises bile salt transport while conferring resistance to HBV/HDV. Understanding these mechanisms opens avenues for the development of novel therapeutics, including smaller drugs or peptidomimetics, that target HBV/HDV binding and infection more effectively.
This collaborative effort represents a significant leap forward in our understanding of HBV/HDV pathogenesis and therapeutic interventions. By deciphering the molecular dance between viral peptides and cellular receptors, researchers are poised to devise more precise and efficacious strategies for combating hepatitis, ultimately reducing the burden of liver-related diseases worldwide.
Nature Communications