Despite modest sales growth in the first half of the current financial year, ASX-listed Nova Eye Medical believes it’s poised for stronger returns from its glaucoma portfolio with a revamped canaloplasty device, making the technology more accessible to a broader base of ophthalmologists.
On 17 February, the ophthalmic medical technology company released its half-year financial results for the six months ended 31 December 2021.
Glaucoma surgical devices – comprising its iTrack minimally invasive glaucoma surgery (MIGS) system and Molteno3 glaucoma drainage device platform – accounted for almost all sales revenue, $6.54 million.
This represented a 1.6% increase in glaucoma surgical device revenue in constant currency terms, compared with the prior comparative period (pcp). EBITDA-level loss from the glaucoma surgical devices segment was $670,000, an improvement from $970,000 in the pcp after allowing for $1.4 million of US Government COVID-19 stimulus during this current reporting period.
The global market value for glaucoma surgical devices is currently estimated at US$610 million (AU$850 m) per year with 17% annual growth.
Despite what it described as “modest sales growth” during the half year ended 31 December 2021, Nova Eye stated it was confident that progress on its next generation canaloplasty device, iTrack Advance, including the commencement of pre-launch activities in Europe, provided an outlook for strong sales growth.
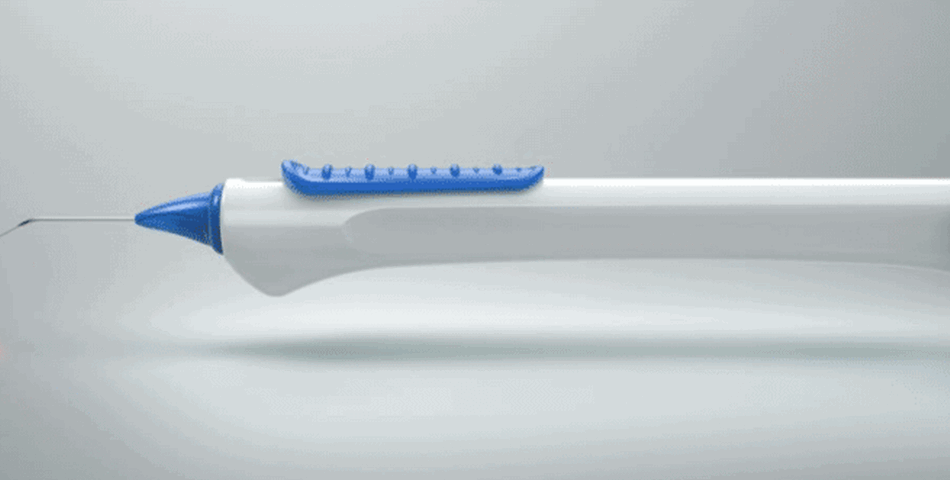
The new iTrack Advance leverages the proprietary features of the original iTrack device but is said to incorporate a new handheld injector design, which improves overall surgical efficiency. The company hopes this improved ease of use will drive procedural adoption by a new, larger demographic of surgeons comprising comprehensive ophthalmologists.
“During the period we commenced a series of pre-launch activities for the new iTrack Advance, including a surgical training program,” Mr. Tom Spurling, managing director of Nova Eye Medical, said
“Several initiatives were executed during the period to drive improved market penetration of the Company’s iTrack™ MIGS device, which resulted in strong sales growth. More importantly, however, these initiatives have set the foundation for further growth in the coming years.”
“This program has initially centered on our existing base of glaucoma professors in Germany and will be followed by outreach to new customers, cataract surgeons and comprehensive ophthalmologists. This will be an exciting milestone in our efforts to drive greater procedural adoption of canaloplasty and thus to generate sales growth.”
Breaking down its latest sales figures, Nova Eye reported that glaucoma surgical device sales in Europe grew by 26% during the period (in constant currency compared with pcp) to US$1.22 million (AU$1.7 m). Sales also grew in China by 44% (in constant currency compared with the pcp) to US$490,000 (AU$683,000). In the US, however, revenue dropped 10% to US$3.04 million (AU$4.2 m).
“While the USA remains our most important market, our direct sales presence in Germany represents an important milestone in our stated growth strategy. We have a rich history in Germany, and it represents one of the most important markets for our glaucoma treatment products
Revenue growth in Europe was primarily due to investments in commercial infrastructure in Germany, including the establishment of a direct sales team. In China, a sustained marketing and sales effort by the company’s local distribution partner resulted in improved sales performance. In the US sales fell due to intensified levels of competition.
Despite this challenge, favorable reimbursement changes from January 1, 2022, have triggered new surgeon interest in canaloplasty, which, combined with the new iTrack Advance canaloplasty device, is expected to drive sales growth and regain market share, Nova Eye stated.
“Following its successful introduction into key territories in Europe over the coming months, a major priority for the business in 2022 will be the US market launch of the iTrack Advance,” Spurling said.
“This device takes the established effectiveness, accuracy and reliability of the original iTrack and combines it with an ergonomic, easy-to-use handheld injector that’s optimized for all ophthalmic surgery and specialist settings. As a result of its improved ease of use, we expect uptake by comprehensive surgeons to be strong. This will facilitate greater patient access to canaloplasty during the earlier stages of the disease process.”
Nova Eye Medical, Glaucoma
The Company made a concerted effort to reset its global glaucoma sales, marketing, clinical and IP infrastructure during the period, with much of this effort focused on the Company’s proprietary iTrack™ MIGS device, which offers substantial opportunity for growth in the burgeoning MIGS market. Significant investments were also made in the Company’s clinical program for iTrack™ during the period, including the initiation of a new multi-center clinical study (“MAGIC”, NCT04769453) to provide prospective clinical evidence in support of the superior effectiveness of iTrack™.
https://www.insightnews.com.au/
Author: Myles Hume