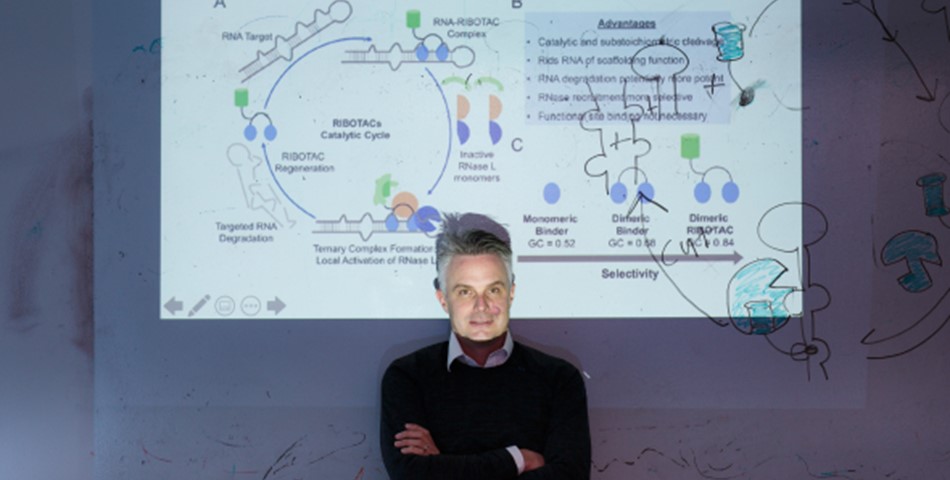
A collaboration including The Wertheim UF Scripps Institute and the Max Planck Institute of Molecular Physiology vastly expands the catalog of potential RNA-targeted medicines.
The cancer gene MYC has been called the “Mount Everest” of cancer research because of the difficulty of designing medications that can disable it, and the expectation that an effective MYC drug could help so many cancer patients. A collaboration among RNA scientists, chemists and cancer biologists in Florida and Germany has climbed that peak, while opening new routes to summit other similarly hard-to-treat diseases.
The researchers describe their strategy today in the journal Nature.
The scientists’ approach directs cells’ recycling enzymes to cancer genes’ RNA and cuts up key segments to prevent them from doing harm. The tactic worked against the MYC cancer gene, and also two other challenging cancer genes, JUN and MIR155. All three of the cancer genes regulate the transcription of other genes, igniting rapid tumor growth.
“For cancer patients whose disease is driven by these common but challenging oncogenes, the RNA degrader approach may offer new hope,” said Herbert Waldmann, Ph.D., director of the Max Planck Institute of Molecular Physiology in Dortmund, Germany.
Their study also opens new possibilities for targeting RNA with medicines, opening potentially many other genetic diseases to this treatment approach, said chemist and institute professor Matthew D. Disney, Ph.D., of The Herbert Wertheim UF Scripps Institute for Biomedical Innovation & Technology and the UF Health Cancer Center.
“We discovered around 2,000 new RNA structures that are able to bind drug-like small molecules, and identified six new chemotypes able to bind RNA,” Disney said. “We basically have created an encyclopedia of druggable RNA folds.”
Among the most challenging of drug targets, MYC is also one of the most important. Its activation may affect 70% or more of all human cancers. It can direct many other genes to be built or silenced. It affects cell growth, and even a cell suicide program that leads damaged cells to self-destruct, a vital process called apoptosis. It also affects the repair process of damaged DNA, and the growth of blood vessels. In many cancers MYC is overexpressed, leading cells to grow and divide too rapidly.
Activation of another cancer gene, JUN, has been seen in more than 20 different cancer types, including glioblastoma, breast, prostate, lung, colorectal cancer and more.
A small RNA gene called MIR155, meanwhile, drives inflammation and the growth and spread of many cancers. Researchers have found it activated in breast, kidney, gastric and other cancers.
Efforts to make drugs that prevent the three oncogenes from doing harm have largely failed, due to their complex structural challenges.
International Collaboration
Surmounting these challenges involved the efforts of an international team of three laboratories:
In Florida, Disney’s group brought insights about drug discovery for RNA structures, and new RNA degradation technologies.
At the Max Planck Institute of Molecular Physiology, Waldmann’s group designs compounds inspired by natural substances, such as those that have produced many antibiotics and cancer drugs.
Organic chemist Frank Glorius, Ph.D., of the Organic Chemistry Institute at the University of Münster in Germany, has developed innovative methods to build new drug-like molecules. Some of his compounds, housed in Waldmann’s collection, were specifically designed to influence biology in cell membranes. Compounds derived from imidazole, a molecule common in natural products and drugs, modified with carbon-based chains, ultimately proved to be the most effective at binding to the cancer-linked RNAs.
“The compounds look a bit like a TV on two long, thin legs,” Glorius said.
“It was a true collaboration,” Waldmann added. “All three of us and our groups were needed to arrive at these insights.”
The idea for the partnership first arose in 2016 during a scientific conference in Spain, when Waldmann and Disney compared notes after a talk. Both agreed that targeting RNA with drugs presented an exciting possible way to target incurable diseases, but it was early days. Nothing like that existed in the market.
Targeting RNA to Stop Cancer
RNA represented a difficult drug-targeting challenge. Comprising just four nucleotides, RNA has many jobs. It reads genes, assembles proteins and is recycled to carry out other work in the cell. Its structures are so diverse and changeable that many in the pharmaceutical industry wrote off trying to make RNA-directed medicines as a pointless exercise.
But over the course of 15 years, Disney and his group have identified many conserved, druggable RNA structures. Disney’s team built their own compound collections and showed in mice that targeting RNAs can wipe out cancer tumors and improve other diseases, including ALS and myotonic dystrophy. RNA offered an alternate route to tackle diseases whose key protein structures couldn’t be reached by medications.
By Stacey DeLoye
Director of Communications for UF Scripps Biomedical Research